Introduction
Acute and chronic graft vs. host disease (a/cGVHD) are major causes of treatment failure and non-relapse mortality (NRM) in multiple myeloma (MM) patients (pts) undergoing allogeneic hematopoietic cell transplantation (allo-HCT). Use of post-transplant cyclophosphamide (PTCy) is now an established method for GVHD prophylaxis after HLA haplo-identical (haplo)-HCT. We previously reported data in MM pts undergoing haplo-HCT (EBMT/CIBMTR) and showed that PTCy was associated with promising overall survival (OS) (Sahebi et al., BBMT 2019). However, data using PTCy for GVHD prophylaxis in other donor types are very limited in MM.
Methods
We evaluated PTCy as GVHD prophylaxis in MM pts who underwent a first allo-HCT using matched related (MRD), matched unrelated (MUD), mismatched related or unrelated (MMRD/MMUD, one antigen), and haplo donors within EBMT centers. OS and progression free survival (PFS) were determined by means of the Kaplan-Meier estimator. Neutrophil and platelet engraftment, relapse and NRM, and a/cGVHD were analyzed individually in a competing risks framework with relapse and death as competing events. Cox proportional hazards regression was used in the multivariable analyses. All estimates include 95% confidence intervals. All included covariates are listed in the Table.
Patient Characteristics
Between 2012-2018, a total of 295 MM pts received PTCy as GVHD prophylaxis. Median age at transplant was 55 yrs. Allo-HCT was given at a median interval of 34.9 mo from MM diagnosis. The conditioning regimen included reduced intensity (RIC, 193, 65.4%) and myeloablative (MAC, 102, 34.6%). All pts except 10 (3.4%) had prior autologous HCT; all of these 10 pts received a haplo-HCT. GVHD prophylaxis included PTCy + cyclosporine A or tacrolimus +/- mycophenolate mofetil in the majority of patients (239, 81%), and this combination was used in nearly every patient with haplo-HCT. Overall, peripheral blood was used as the stem cell source in about 80% of pts, but bone marrow was used in nearly 40% of haplo-HCTs.
Results (Median and 95% confidence interval are provided)
1) Median time to neutrophil engraftment was 19 d (18-19 d) with no apparent difference among donor types.
2) Median time to platelet engraftment was 23 d (21-26 d), whereas haplo-HCT engraftments were longer (27 d (25-33 d)).
3) NRM at 1 yr was 18% (12-22%) and at 2 yrs was 19% (14-24%), with no significant difference among different donor types. Age < 50 yrs significantly decreased NRM to 9% (2-16%, p=0.027) at 2 yrs.
4) Cumulative incidence of grade II-IV aGVHD at +100 d was 30% (25-36%), and 1 yr cGVHD was 27% (21-32%), with no apparent difference among donor types.
5) OS was 63% (57-69%) at 1 yr and 51% (45-58%) at 2 yrs for the whole group, with no statistical difference among different donor types after a median follow-up of 26.1 mo. Disease status at transplant < PR significantly decreased OS to 35% (22-47%, p=0.005) at 2 yrs.
6) PFS was 42% (36-49%) at 1 yr and 26% (20-32%) at 2 yrs for the whole group without apparent significant difference among donor types. Disease status at transplant < PR significantly decreased PFS to 16% (6-26%, p=0.028) at 2 yrs.
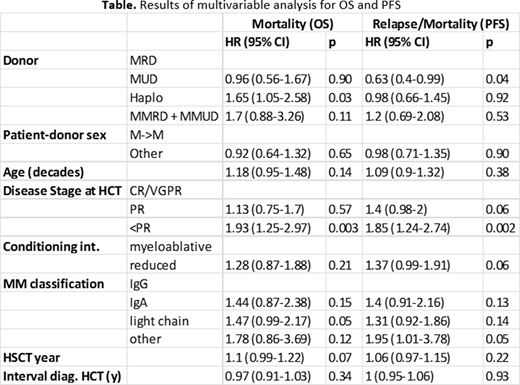
7) However, in multivariable analyses, donor type using haplo, HR 1.65 (0.56-1.67, p=0.03), was associated with increased mortality in addition to disease status at allo-HCT < PR, HR 1.93 (1.25-2.97, p=0.003). Finally, MUD was associated with an improved PFS, HR=0.63 (0.4-0.99, p=0.04). Disease status < PR, HR=1.85 (1.24-2.74, p=0.002) was again associated with inferior PFS.
Summary
PTCy in MM patients undergoing allo-HCT throughout donor types resulted in a low incidence of aGVHD grade II-IV of 30% and cGVHD of 27%, with OS of 51% and PFS of 26% at 2 yr. Our first donor comparison using PTCy revealed improved survival in matched (MRD and MUD) versus haplo allo-HCT after adjusting for other risk factors.
Blaise:Jazz Pharmaceuticals: Honoraria. Tarella:TG-therapeutics: Research Funding; ImmunoGen: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. McDonald:venetoclax advisory board in South Africa (in CLL context): Consultancy; Alberts Cellular Therapy: Current Employment. Milpied:Roche: Honoraria, Other: Travel support; Astellas: Honoraria; Gilead Sciences: Other: consultancy or advisory role; Celgene: Other: Travel support; Sandoz: Honoraria, Other: consultancy or advisory role; Janssen: Honoraria. Yakoub-Agha:Celgene: Honoraria; Novartis: Honoraria; Gilead/Kite: Honoraria, Other: travel support; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria. Schonland:Janssen: Honoraria, Other: travel support to meetings, Research Funding; Prothena: Honoraria, Other: travel support to meetings, Research Funding; Takeda: Honoraria, Other: travel support to meetings, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal